
2023-09-27
As medical technology advances and public demand for healthcare grows, the development of home ECG monitors has garnered significant attention. However, achieving medical-grade standards for these devices has remained a challenge in the industry. On September 27, 2023, BAHEAL Pharmaceutical (301015.SZ) introduced a groundbreaking product: the BAHEAL® Heartpal Home ECG Device, a 12-lead home ECG monitor capable of timely detecting myocardial ischemia, providing crucial time for treatment in heart attack patients.

In recent years, with rising living standards and lifestyle changes, the incidence and mortality rates of cardiovascular diseases have been steadily increasing. According to the “China Cardiovascular Health and Disease Report 2022,” there are approximately 330 million cardiovascular disease patients in China, with cardiovascular diseases accounting for over 40% of all disease-related deaths. Each year, more than 1 million new myocardial infarction cases are reported, with a treatment rate of less than 5%. For cardiovascular patients, ECG monitoring is a vital tool for maintaining daily health and early detection of conditions, crucial for managing heart attacks within the "golden two hours."
The BAHEAL® Heartpal Home ECG Device meets medical-grade ECG standards, offering diagnostic value comparable to hospital equipment. It is designed for out-of-hospital ECG monitoring for cardiovascular patients and high-risk individuals. Unlike single-lead or three-lead products, the BAHEAL® Home ECG Device not only detects arrhythmias but also monitors myocardial ischemia and myocardial infarction, providing essential time for intervention. The device has obtained a national invention patent and has been certified as a Class II medical device.
The BAHEAL® Heartpal Home ECG Device is user-friendly; it simply clips onto the chest and can be operated by hand. In less than a minute, users can obtain ECG data, with alerts for any abnormalities. Through the "BAHEAL ECG" app, users can access 24/7 real-time consultations with online doctors or receive ECG diagnostic reports verified by top hospital ECG specialists via the "Doctor Image Interpretation" function.
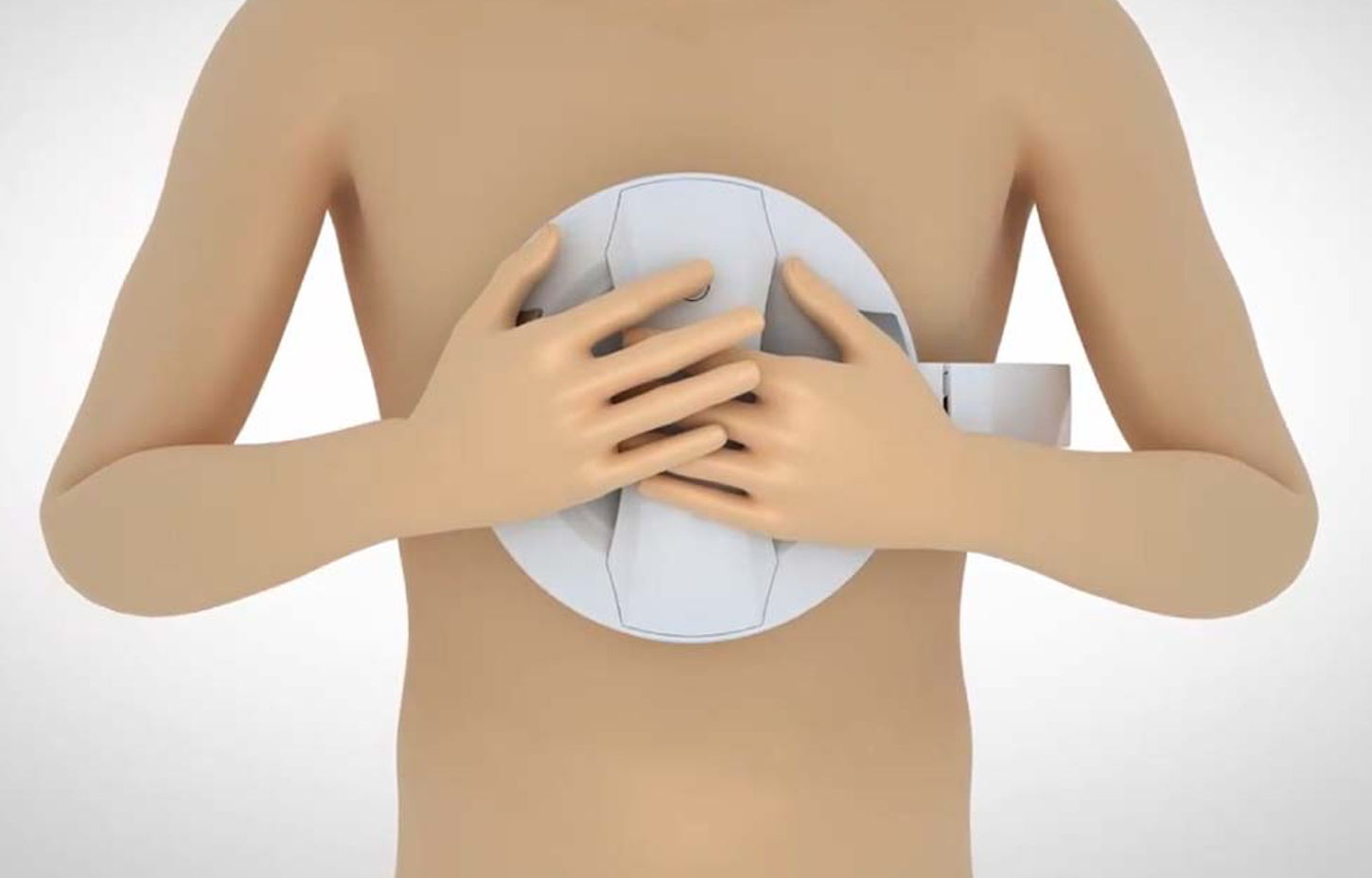
Speaking on the launch, Zhang Yuan, Vice President of BAHEAL Medical, emphasized that the company’s mission is to optimize medical scenarios through technological innovation. The BAHEAL® Heatpal Home ECG Device embodies this mission, offering timely monitoring of myocardial ischemia and providing early disease warnings for high-risk cardiovascular patients. Ensuring timely and effective treatment for heart attack patients at critical moments is not only BAHEAL Pharmaceutical’s goal but also the collective objective of the healthcare industry.
As a professional third-party commercialization platform in the pharmaceutical industry, BAHEAL Medical has successfully operated a number of well-known brands such as D-Cal, Mite, and Hylo. In recent years, the company has expanded its presence in OTC and healthcare, OTX prescription drugs, oncology, critical care drugs, and high-end medical devices. In the cardiovascular medical device field, BAHEAL also manages AtriCure’s atrial fibrillation treatment devices and BrioHealth’s fully magnetic levitation artificial heart. The launch of the BAHEAL® Heatpal Home ECG Device is not only a proactive move in the home medical device sector but also an important extension of BAHEAL’s product lineup in cardiovascular disease management.
To mark the 24th "World Heart Day," BAHEAL Pharmaceutical, in collaboration with Weibo Health, launched the "Heart Protection Campaign," offering the public a series of cardiovascular disease education and free experience services. Protecting lives begins with the heart!
